Nitrogen Control in MIM
Introduction
Nitrogen can combine with molybdenum and manganese, then replace nickel as the austenitic formation element effectively, and increase the corrosion resistance finally. Nitrogen element can be added in austenitic stainless steel through the sintering atmosphere.
N is a neutral and beneficial element for most ferrous alloys, it is an interstitial to increase strength same as carbon element. Sintering atmospheres with nitrogen will reduce cost and flammability.
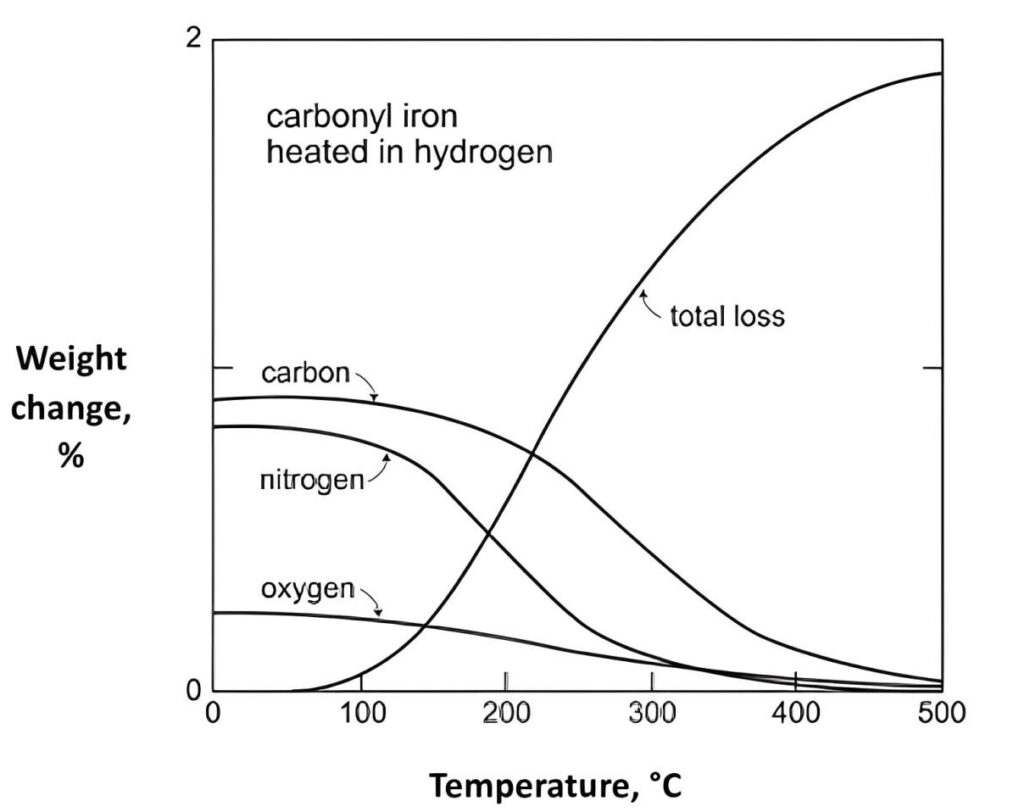
Sintering under nitrogen atmosphere is the key step to improve mechanical properties by solution hardening mechanism, due to the interstitial nitrogen in final sintered parts. In addition, limited cooling rate in furnace will lead to precipitation of chromium nitride, it has significant influence on ductility, while little on the yield and ultimate strength.
Normally, the nitrogen level must below 0.95%, in order to avoid the ductile-to-brittle transition in final properties.
Nitrogen Effect in MIM Sintering
Austenite Forming Element
N (Nitrogen) is a strong forming elements in austenite. Nitrogen can replace the nickel elecment in steel, and improve the mechanical properties and corrosion resistance significantly. The high-nitrogen steels have greater than 0.08 wt.% nitrogen in the martensitic matrix, or more than 0.4 wt.% nitrogen in the austenitic matrix.
PANACEA is the common high-nitrogen and nickel-free stainless steel in MIM process. Comparing with traditional melting process, high nitrogen steel in MIM process are more beneficial. Once under the solubility limit of nitrogen 0.9 wt.%, there is no effect on the good ductility and toughness properties. Otherwise, Cr2N are formed once exceed the solubility limit, this leads to decrease of ductility, corrosion resistance and toughness.
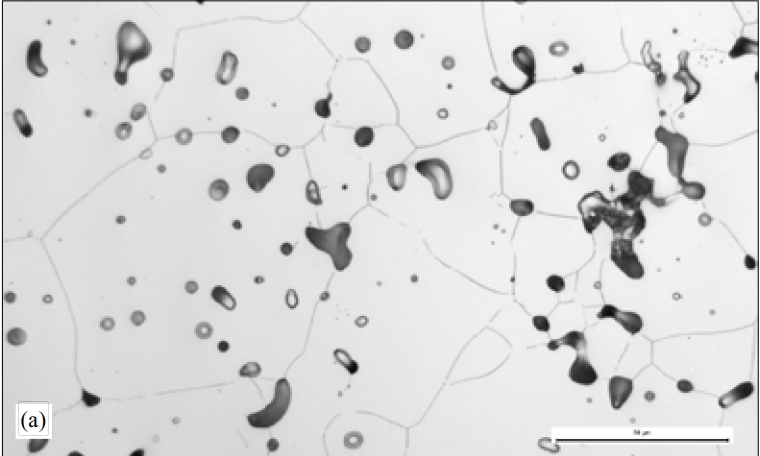
SS316L Sintering in Nitrogen
Austenite Stabilization
In addition, nitrogen stabilizes the austenitic phase strongly. Nitrogen-ally austenitic stainless steel can be hardened to very high strength level without strain-induced martensite phase formation. Nitrogen increases the resistance against undesired δ-ferrite, then suppresses the formation of martensite during formation.
SS316L Sintering in Cracked Ammonia
Except nitrogen, there are other potential elements for nickel replacement and austenite stabilization.
- Cobalt: its allergeologic behaviour makes it not consider as the replacement for nickel.
- Carbon: it has negative influences on toughness, nitrogen solubility, corrosion resistance.
- Manganese: it may be biocompatible.
- Molybdenum: it enhances nitrogen solubility, and increase localized corrosion resistance. But high molybdenum contents will result in formation of δ-ferrite and σ-phase, and finally decrease toughness and corrosion resistance.
- Chromium: similar to molybdenum, high content will decrease toughness and corrosion resistance.
Strength Effect
Nitrogen content affect the final values for yield strength(YS), ultimate tensile strength (UTS) and elongation (A). In a nitrogen sintering atmosphere, a large amount of interstitial nitrogen can improve the mechanical properties through the solution hardening mechanism. However, limited cooling rates will lead to precipitation of chromium nitride, this leads to little influence on yield and ultimate strength, but significant decrease of ductility. Normally, the nitrogen level should be kept under 0.95%, in order to avoid the ductile-to-brittle transition.
Nitrogen in MIM 420
Nitrogen has low solubility in martensitic matrix, almost no N are detected in sintered 420 specimens, no matter from top to bottom area. Most nitrogen atoms are diffusing effectively into 420 alloy with formation of carbo-nitride Nb particles, not the N dissolvation in the martensitic matrix. Nb is a strong affinity former for nitrogen, which is higher than Cr. Therefore, 420 can keep an effective content of chromium after sintering, this is benefical for corrosion resistance.
Nitrogen in SS430
SS430 in N2 sintering has higher strength level than that in H2. This is clearly that no relation to difference in porosity, nitride formation in sintering is the key factor. In H2 sintering, there is significant loss of nitrogen, this result in lower mechanical properties, while nitrogen level in N2 sintering is stable.
Nitrogen in SS316L
Nitrogen adsorption of SS 316L occurs when sintering in N2 atmosphere, the fine alloy powders adsorb the most nitrogen at the surface area. This leads to the highest strength of SS 316L with the lowest ductility. This nitrogen adsorption normally promotes the formation of austenite at key boundary diffusion zones, then retards the diffusion processes, and impedes densification finally.
Sintering 316L in cracked ammonia has the similar trends as in N2, while achieve the higher densities. The hydrogen from cracked ammonia has reducing effect, this will assist in desificaiton.
MIM 17-4 PH
In stainless steel 17-4 sintering, the nitrogen is soluble in austenite, and forms Cr2N precipitates in cooling. Therefore, cooling at 200°C/min below 900°C is applied to suppress chromium nitride formation. 17-4 PH can achieve the highest ductility and tensile strength in nitrogen sintering, while achieve the highest yield strength and corrosion resistance in hydrogen sintering.
PANACEA
In nickel-free stainless steel (PANACEA), nitrogen can combine with molybdenum and manganese effectively, it can replace nickel as the austenite formation element, and increase the corrosion resistance. Normally, the high nitrogen austenitic stainless steel are added by the sintering atmosphere. Sintering under nitrogen atmosphere is also the key step to improve mechanical properties, a large amount of interstitial nitrogen result in the solution hardening. In addition, limited cooling rates in the furnace will lead to precipitation of chromium nitride, which has significant reduction in ductility, but little influence on yield and ultimate strength.
Tool Steels
All tool steels are sintered in nitrogen atmosphere, these tool steels will reach full density of 99.6% at 1240°C to 1360°C. Nitrogen element has stabilizing effect on austenite, and increase the liquidus temperature in tool steels. Furthermore, the C will be replaced by N in nitrogen sintering.
Conclusion
Nitrogen is the key element in MIM sintering. ZCMIM has more than 15 years of experience in different alloys. We can control N-content in every MIM project for required applications. Contact us for your next MIM project.